liquid
What is a liquid?
A liquid is a type of matter with specific properties that make it less rigid than a solid but more rigid than a gas. A liquid can flow and does not have a specific shape like a solid. Instead, a liquid conforms to the shape of the container in which it is held. Although this is similar to a gas, a liquid does not expand to fill up the container like a gas. Examples of liquids at room temperature (about 20 degrees Celsius or 68 degrees Fahrenheit) include water, oil, alcohol and mercury.
The term liquid can refer to the type of substance or to its state of matter. Water, for example, is the most common liquid on Earth and, in its liquid state, covers a substantial percentage of the surface. However, it is in a liquid state only between the temperatures of 0 degrees Celsius (32 degrees Fahrenheit) and 100 degrees Celsius (212 degrees Fahrenheit). At lower temperatures, it transitions to a solid state and becomes ice (frozen water). At higher temperatures, it transitions to a gaseous state and becomes water vapor.
Whether frozen or vapor, the molecular structure of water remains the same as in its liquid state. Every form is still water, just in different states of matter. However, frozen water and water vapor are not liquids, nor are they in a liquid state.
When matter is in a solid state, the atoms or molecules are packed together more tightly than matter in the liquid state, but the difference between their densities is relatively small, just enough so the molecular entities in a liquid can move around each other. In a gas, the molecular entities are spread much further apart and move and expand more freely within their available space.
When a liquid is heated, the molecular entities gain kinetic energy. If the temperature rises enough, the liquid becomes a gas or reacts with chemicals in the environment. For example, water becomes gaseous when it is heated gradually, but alcohol can combust when combined with oxygen if heated suddenly and dramatically. When a liquid is cooled, the molecular entities lose kinetic energy. If the temperature becomes low enough, the liquid becomes a solid.
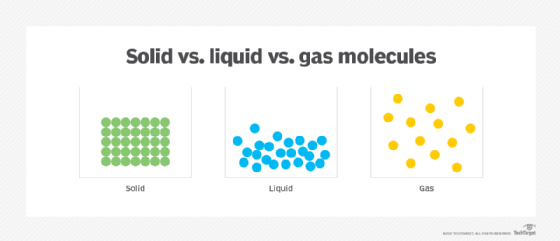
What are characteristics of a liquid?
Liquids can vary substantially from each other. For example, olive oil weighs more than vinegar and is much thicker, causing it to pour more slowly. Olive oil also starts to solidify around 12 degrees Celsius (54 degrees Fahrenheit), whereas vinegar starts to freeze at around -2.2 degrees Celsius (28 degrees Fahrenheit). Despite the differences between liquids, they can generally be characterized by a set of common physical properties:
- Cohesion. The molecular entities that make up a liquid attract each other to varying degrees due to the intermolecular forces that bind them together. Cohesion can be seen in a liquid's surface tension, which is what holds water together in drops or makes it possible to float a pin on its surface.
- Adhesion. Attractive forces can exist between a liquid and another substance to varying degrees, depending on the type of liquid and the other substance. This explains why water clings to surfaces in different ways, such as glass compared with plastic. Adhesion also explains capillary action, the tendency for liquid to ascend narrow cylinders or permeable substances, as when a nurse uses a narrow glass tube to collect a blood sample from a patient.
- Volume. Although liquid conforms to the shape of its container, it maintains a relatively fixed volume. A change in pressure or temperature might alter the volume slightly, but as a whole, the volume remains fairly consistent, unless affected by vaporization or evaporation. For example, if a full carton of milk is exactly 1 liter in volume, the milk has the same volume even if you pour it into a 20 L vat. This behavior is much different from gas, which expands to fill its container.
- Compressibility. Strong intermolecular forces hold liquids together, similar to solids, resulting in a highly dense substance that makes a liquid fairly incompressible, another behavior much different from gas.
- Shapelessness. A liquid has no fixed shape. Like gas, it takes the shape of the container that holds it, except that it does not expand to fill the container like gas.
- Viscosity. A liquid's ability to flow is one of its fundamental characteristics. However, the degree to which it flows depends on its viscosity, which varies according to its molecular size and intermolecular forces. For example, motor oil has a much higher viscosity than water due to its larger molecular structure. Therefore, motor oil flows much more slowly than water.
- Evaporation. Because the molecular entities in a liquid move around a great deal, they often collide with each other or with the container holding the liquid. These collisions cause energy to be transferred between molecular entities. When enough energy is transferred to the liquid's surface, it can break the bonds of surface tension, causing the liquid to evaporate.
What are mixtures?
Liquids are often combined to create mixtures. A mixture is either heterogeneous or homogeneous. A heterogeneous mixture is a combination of substances that do not react chemically with each other. The individual materials are not distributed uniformly, and they retain their individual physical properties, as in the case of oil and vinegar.
A homogeneous mixture, also referred to as a solution, is a type of mixture in which the substances are uniformly distributed and undergo a chemical change. Typically, one substance dissolves into the other to form a new substance with a uniform composition. For example, vodka is a solution made of ethanol and water.
See also: state of charge, Standard temperature and pressure, impedance, coulomb, water cooling, matter, compound, proton, neutron, dielectric material and conductor.
